Search from website
Search from website
Home Technologies IcoCell Technology
IcoCell is a proprietary cell line development platform to efficiently generate CHO-based stable high-performance cell lines. These serve for large-scale GMP production of therapeutic recombinant proteins and monoclonal antibodies, both for clinical trial materials as well as for industrial-scale commercial production afterwards.
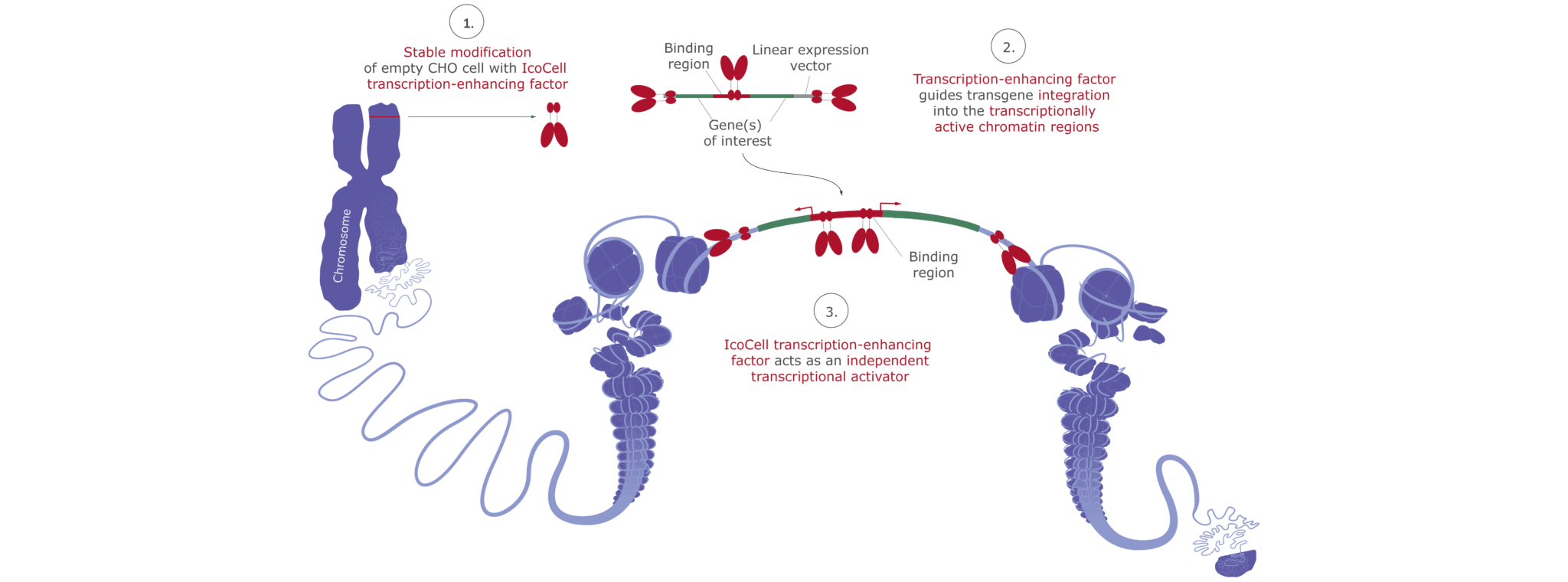
The IcoCell platform is based on a proprietary, fully biosafety-tested CHO-S starter cell line, which is stably modified with a regulatory approved viral transcriptional enhancer. This IcoCell modification ensures the transgenes’ semi-directed and stable integration into transcriptionally active open chromatin, yielding controlled, strong, and lasting protein expression levels.
From Antibody Discovery to Stable CHO Cell Line Development and in-house GMP Manufacturing:
One Site. One Team. One Project. Saving Time, Costs, and Nerves.
Icosagen’s automated single-cell cloning cell line development workflow combines gentle single-cell printing with in-well droplet and whole-cell imaging. The VIPS™ System ensures that every production cell line is a true mono-clone derived from one single progenitor cell. The VIPS™ (Verified In situ Plate Seeding) automatically captures and documents the journey from a single cell to colony outgrowth into a comprehensive clonality report. High-clarity images and accompanying data provide a unique ‘double lock’ of clonality assurance of 99.9% for IND submission.
Additional safety method to the automation-based VIPS™-based clonality report, a further routine visual inspection of the images by experienced CLD scientists assures that every cell line was indeed derived from one single cell.
Step 1. Single-cell seeding evidence
Cells are imaged in multiple z-stack layers in the dry empty well before intelligence-based image analysis confirms the existence of a single cell.
The well is then carefully filled with medium. Step 2. Clonal growth phase
During the clonal growth phase, VIPS™ performs daily high-clarity whole-well imaging, recording a timeline of well images and at the same time, performing confluency analysis. Successful clonal outgrowth can so be traced back to a single cell to confirm monoclonality. The IcoCell technology is a proprietary platform to develop biopharmaceutical high-producer clones in CHO cells, with competitive titers and timelines, and robust, predictive large-scale manufacturing properties for therapeutic proteins and antibodies.
We have designed and optimized the entire Icosagen workflow and IcoCell technology platform such that our clients’ product candidates transition seamlessly and rapidly from the discovery and lead candidates’ stage into a clinical development path, ending with GMP-produced clinical trials drug substance.
IcoCell CHO Cell Line Development and Biosafety Testing
The “empty” CHO IcoCell is genetically stably modified with the regulatory accepted IcoCell transcriptional activator molecule, and afterwards passed a rigorous MCB biosafety testing procedure, as conducted for any completed MCB (Master Cell Bank).
Optimized Gene Expression in IcoCell CHO Cell Lines for Maximal Yield and Quality
For every new-to-be-created IcoCell CHO cell line, the entire gene for the encoded mAb/protein of interest is first optimized for maximal yield and product quality. During IcoCell cell line development, the transgene’s DNA from the the proprietary IcoCell-corresponding expression vector is transfected into the empty starter cell. The IcoCell transcriptional activator mediates the optimal, semi-directed integration of the transgene sequences into the active open chromatin in the CHO chromosomes.
The Role of IcoCell Transcriptional Activator – Integration and Prevention of Transgene Silencing
The robust, regulatory accepted IcoCell transcriptional activator fulfills two tasks at once. First, in a concerted action with corresponding activator binding sites on the IcoCell expression vector, it facilitates the semi-directed integration of the transgene expression cassette into transcriptionally active regions of the CHO host cells’ chromatin, thereby ensuring the controlled and strong expression of the recombinant antibody/protein product. Secondly, the IcoCell transcriptional activator prevents the transgenes’ transcriptional silencing during clonal cell line development and later industrial-scale commercial GMP manufacturing. This way the proprietary IcoCell technology also guarantees the long-lasting expression of the protein of interest over many population doublings, even in commercial thousands of liters bioreactor scales.
Upstream and Downstream Process Optimization For Product Quality and Yield
During upstream and downstream process development, the protein production conditions are further optimized for yield and quality, yet with a close eye on the economic and regulatory aspects of all process steps developed and materials & consumables employed.
Optimization parameters include e. g. high viable cell densities, cell growth rates, high-performance metabolic parameters, bioreactor occupation times, as well as impurities-removement, product retainment, etc., all specific to the relevant protein of interest for continuous and optimal product quality and yields.