Search from website
Search from website
Imagine if a simple test could save your life. For the millions of people living with human immunodeficiency virus (HIV) worldwide, this is not just a hypothetical scenario - it's a reality. Early detection of HIV infection is crucial for effective treatment and prevention of transmission. In this article, we'll explore the science behind HIV testing and how this deadly virus has been turned into a powerful tool for research and therapy.
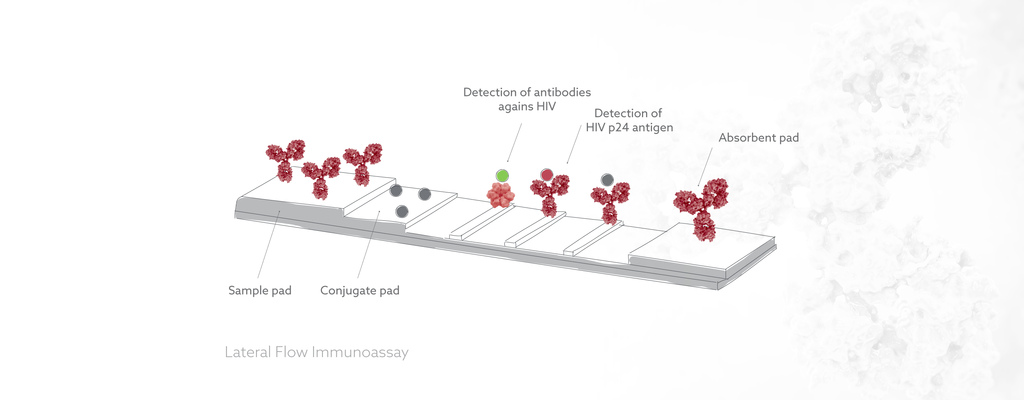
Human immunodeficiency virus (HIV) is a significant global health concern. According to WHO 38 million people worldwide are currently living with HIV. Approximately 40 million have died from AIDS-related illnesses (1), making it one of the largest pandemics since the 1918 Spanish flu outbreak. Early detection of HIV infection is critical for effective treatment and prevention of transmission. HIV infection has a latent period during which no symptoms may be visible, highlighting the importance of regular testing for early detection. As the infection progresses and CD4+ T cell numbers decline below a critical level, the individual consequently develops acquired immunodeficiency syndrome (AIDS) and becomes susceptible to opportunistic pathogens.
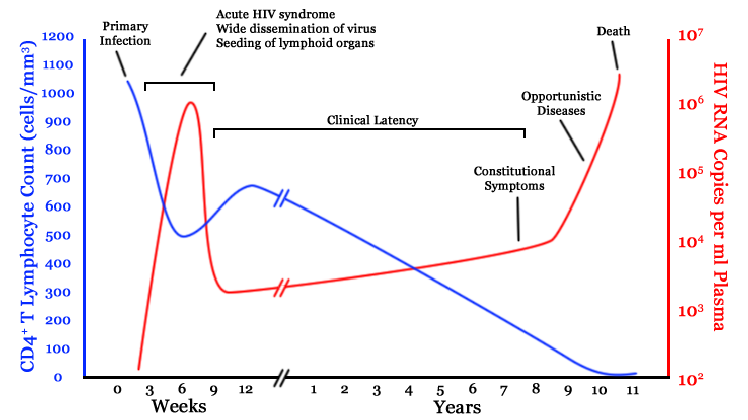
While rapid tests for HIV antibodies are widely available, they may not detect the virus in the early stages when the viral load is still low. That's where p24 antigen comes in. The p24 antigen is a protein that makes up most of the HIV viral core and is produced by the virus shortly after infection. It can therefore be detected in the blood before HIV antibodies are produced. At Icosagen, we offer antibodies against HIV p24 antigen for early detection of HIV infection. Our p24 antibodies can complement rapid HIV tests and improve the accuracy of HIV diagnosis, especially in the early stages. By detecting both HIV antibodies and p24 antigen, fourth-generation combination tests provide more reliable results and can help to start the treatment in time. Interested in improving the accuracy of HIV diagnosis?
Check out our selection of HIV p24 antibodies here.
The accuracy of fourth-generation HIV tests is very high, with sensitivity rates near 100% and specificity rates over 97% (3).
There are several types of HIV tests that are used for diagnosing HIV infections. The main types of tests are antibody tests, antigen tests, and nucleic acid tests (NATs), which include both PCR (polymerase chain reaction) and viral load tests.
Antibody Tests: These tests detect antibodies produced by the immune system in response to HIV infection. The antibodies typically take 2-8 weeks to become detectable after infection, so antibody tests are not effective during the window period (the time between infection and the development of detectable antibodies). Fourth-generation HIV combination tests are more sensitive than antibody tests because they also detect the p24 antigen, which is produced by the virus during the window period.
Antigen Tests: These tests detect the p24 antigen produced by the virus shortly after infection, before antibodies are produced. They are more effective during the window period but are less sensitive than fourth-generation tests because they do not detect antibodies. Antigen tests are also not commonly used for HIV diagnosis.
Nucleic Acid Tests (NATs): These tests detect the genetic material (RNA) of the virus in the blood. NATs are the most sensitive type of HIV test and can detect the virus within days after infection. However, they are more expensive and time-consuming than fourth-generation tests. NATs are therefore typically only used for specialized situations, such as monitoring the viral load in individuals on antiretroviral therapy.
In summary, fourth-generation HIV combination tests are more sensitive than antibody tests and more widely used than antigen tests or NATs for HIV diagnosis. They are effective in detecting HIV infections during the window period and can provide rapid results, making them a valuable tool in HIV prevention and care. However, as with all HIV tests, they are not 100% accurate, and confirmatory testing may be necessary in certain situations.
In some countries, HIV self-testing is not allowed or strictly regulated, and only national laboratories or authorized healthcare facilities can supply them. One reason for the regulation of HIV self-tests is to ensure the accuracy and reliability of the results. Some self-tests may not be as sensitive or specific as laboratory-based tests, which could lead to false positives or false negatives. Another concern is the potential for harm caused by inaccurate or misinterpreted results, which could lead to stigma, discrimination, or inappropriate treatment.
However, HIV self-testing can increase access to testing and reduce barriers to HIV prevention and care. Self-testing promote privacy and confidentiality, and reach populations that may be hesitant to access traditional healthcare services.
The World Health Organization (WHO) recommends that countries consider offering HIV self-testing as an additional option for testing. However, it also emphasizes the need for appropriate regulations, quality assurance, and linkage to care for those who test positive. The WHO has also developed guidelines on HIV testing and counseling that address ethical considerations, confidentiality, and informed consent.
The nearest place to get tested and treated for HIV infection can be found:
https://www.testfinder.info/ (Europe)
Find out additional information about living with HIV:
HIV has become a valuable tool in research, diagnostics, and therapeutics beyond its use in developing treatments for HIV infection itself. One application is the study of immunology. HIV affects the immune system and can therefore help researchers gain a better understanding of how the immune system works. Additionally, in diagnostics, HIV is used as a model virus to develop and test new diagnostic tools and techniques. These tools can then be applied to other viral infections. Researchers are using HIV as a model system to test new drugs and vaccines against other viruses, such as Ebola and Zika. Overall, the study of HIV has contributed to advancements in several areas of medical research and practice.
VLPs are protein structures that mimic the overall structure of viruses, but lack the viral genome. They are engineered by expressing the viral structural proteins (such as Gag, Pol, and Env) in a host cell. The proteins then self-assemble into particles that resemble the HIV virion. The resulting VLPs can be used for various purposes, as described below.
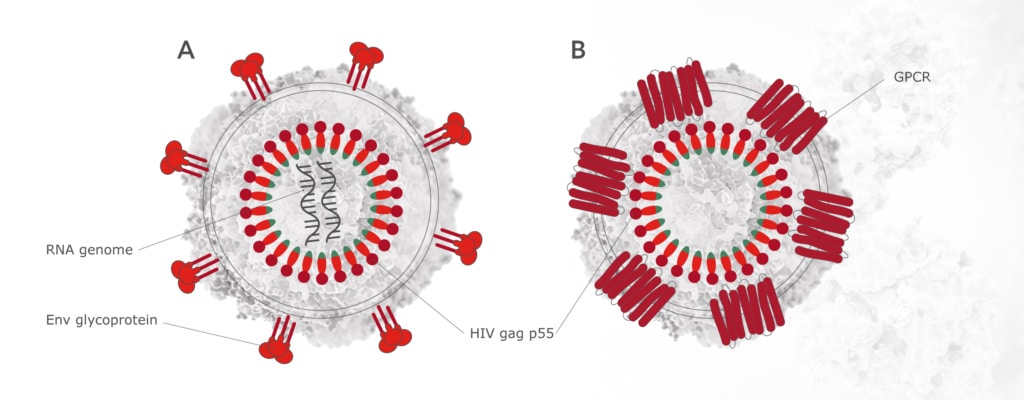
In addition to HIV tests, researchers have used Icosagen's p24 antibodies in purifying these virus-like particles (VLPs). VLPs have diverse applications as they can be used
Due to the numerous applications of VLPs, the demand for them has significantly increased. However, it is usually difficult to obtain pure VLPs and to scale up the process (6). In the next paragraphs we introduce some methods for doing so.
Icosagen’s p24 antibody has been used to show the efficiency of purifying HIV VLPs using ion-exchange monoliths (5), hydroxyl-functionalized monoliths (6) and polymer-grafted chromatography (7). P24 antibody has also been used to compare the methods (NTA, SE-HPLC, MALS) for characterizing VLPs (8).
Firstly, in the article "Purification of HIV-1 gag virus-like particles and separation of other extracellular particles" by Steppert, Burgstaller et al. a chromatography method for purifying HIV-1 gag virus-like particles was described (5). In this method ion-exchange monoliths were used. The method is faster and more cost efficient than density gradient centrifugation and can be scaled up.
Secondly, in the article "Separation of HIV-1 gag virus-like particles from vesicular particles impurities by hydroxyl-functionalized monoliths", Steppert, Burgstaller et al. describe another novel approach to separating HIV-1 VLPs from other extracellular vesicles and impurities (6). The authors used a hydroxyl-functionalized monolith, a type of porous material with a high surface area, to selectively capture and isolate HIV-1 gag VLPs. Comparatively to ultracentrifugation and size exclusion chromatography, the monolith provided superior selectivity and purity.
Thirdly, Patricia Pereira Aguilar, Tobias Amadeus Schneider, Alois Jungbauer et al. showed in “Polymer-grafted chromatography media for the purification of enveloped virus-like particles, exemplified with HIV-1 gag VLP” that polymer-grafted chromatography media can be used to selectively purify HIV-1 gag VLPs from complex biological samples (7). This method allows a flexible scale-up and direct loading of cell culture supernatants. Additionally, it could easily be adapted for the purification of other VLPs.
Finally, in the article "Quantification and characterization of virus-like particles by size-exclusion chromatography and nanoparticle tracking analysis" Steppert, Burgstaller, Jungbauer et al. used three methods to characterize VLPs. These methods were nanoparticle tracking analysis (NTA), size-exclusion high-performance liquid chromatography (SE-HPLC) with UV detection, and detection with multi-angle light scattering (MALS) (8). The study demonstrated that these techniques are useful for characterizing VLPs. The methods also allow for rapid and automated quantification of HIV-1 gag VLPs in various sample matrixes at different purity stages.
In all of these four articles the cell culture (CHOEBNALT85 cell line) and HIV p24 antibodies were provided by Icosagen. You can find Icosagen's selection of HIV antibodies at this link.
At Icosagen, we are committed to advancing HIV research by providing reliable solutions for HIV detection. Stay tuned for more updates on our p24 antibodies and related research!
1 - https://www.who.int/data/gho/data/themes/hiv-aids
2 - Pantaleo, G., Graziosi, C., & Fauci, A. S. (1993). The immunopathogenesis of human immunodeficiency virus infection. The New England journal of medicine, 328(5), 327–335. https://doi.org/10.1056/NEJM199302043280508.
3 - Han, H., Huang, Y., Dong, Q., Huang, Y., Lu, J., Wang, W., & Chen, K. (2019). Clinical Application Evaluation of a Fourth-Generation HIV Antigen Antibody Combination Screening Assay. Clinical laboratory, 65(10). https://doi.org/10.7754/Clin.Lab.2019.190220
4 - Staufenbiel, M., Wiederhold, K., Tissot, A. C., Frey, P., Fulurija, A., Hiestand, P., Jennings, G. T., Lüönd, R., Mayer, P., Ortmann, R., Stoeckli, M., Stumper, B., Zurini, M., Mir, A., Bachmann, M. F., & Christoph, W. (2006). Immunization with Aβ1-6 coupled to the virus-like particle Qβ (CAD106) efficiently removes β-amyloid without inducing Aβ-reactive T-cells. Alzheimer's & Dementia, 2(Suppl 1), S20-S20. https://doi.org/10.1016/j.jalz.2006.05.061
5 - Steppert, P., Burgstaller, D., Klausberger, M., Berger, E., Aguilar, P. P., Schneider, T. A., Kramberger, P., Tover, A., Nöbauer, K., Razzazi-Fazeli, E., & Jungbauer, A. (2016). Purification of HIV-1 gag virus-like particles and separation of other extracellular particles. Journal of chromatography. A, 1455, 93–101. https://doi.org/10.1016/j.chroma.2016.05.053
6 - Steppert, P., Burgstaller, D., Klausberger, M., Kramberger, P., Tover, A., Berger, E., Nöbauer, K., Razzazi-Fazeli, E., & Jungbauer, A. (2017). Separation of HIV-1 gag virus-like particles from vesicular particles impurities by hydroxyl-functionalized monoliths. Journal of Separation Science, 40(4), 979-990. https://doi.org/10.1002/jssc.201600765.
7 - Pereira Aguilar, P., Schneider, T. A., Wetter, V., Maresch, D., Ling, W. L., Tover, A., Steppert, P., & Jungbauer, A. (2019). Polymer-grafted chromatography media for the purification of enveloped virus-like particles, exemplified with HIV-1 gag VLP. Vaccine, 37(47), 7070–7080. https://doi.org/10.1016/j.vaccine.2019.07.001
8 - Steppert, P., Burgstaller, D., Klausberger, M., Tover, A., Berger, E., & Jungbauer, A. (2017). Quantification and characterization of virus-like particles by size-exclusion chromatography and nanoparticle tracking analysis. Journal of Chromatography A, 1487, 89-99. https://doi.org/10.1016/j.chroma.2016.12.085