Search from website
Search from website
Rapid passive immunity via inhalation - a novel approach against respiratory pathogens
Inhaled Immunity!
The COVID-19 pandemic represents a global challenge that has affected and is expected to continue to affect the lives and health of people across the world.
The distribution of vaccines has brought the relief that everyone was hoping for. However, exceptions apply, and we still need effective treatments to make infections less dangerous and not as severe.
According to the recent report (August 2023) from the World Health Organization, the contagious spread of the SARS-CoV-2 virus has been relatively calm compared to previous years when the infected rate reached an all time high, reporting over 44 million infected.
Globally, as of 30th of August 2023, there have been 770,085,713 reported cases of COVID-19, including 6,956,173 deaths. As of 26 of August 2023, 13,499,983,736 doses of vaccine have been administered.
Figure 2. Confirmed continent COVID-19 cases, sourced by World Health Organization (WHO) as of 31 August 2023.
As the flu season is about to start and the trendline for the infected has shown to rise during colder periods, it’s important to take precautions.
But masks and disinfectants are only tools for momentary relief, not sustainable solutions.
In addition to SARS-CoV-2, there is potential threat for other emerging lethal respiratory viruses that may impact human well-being. Viruses are constantly evolving and technology to combat these changes is becoming more difficult.
As a result of the 2020 pandemic, there has been a swift escalation in the demand for creative resolutions within the biopharmaceutical sector.
Ironically, SARS-CoV-2 has helped medicine evolve more rapidly than ever before, as virtually every pharmaceutical and diagnostics company assembled teams to solve the COVID-19 crisis.
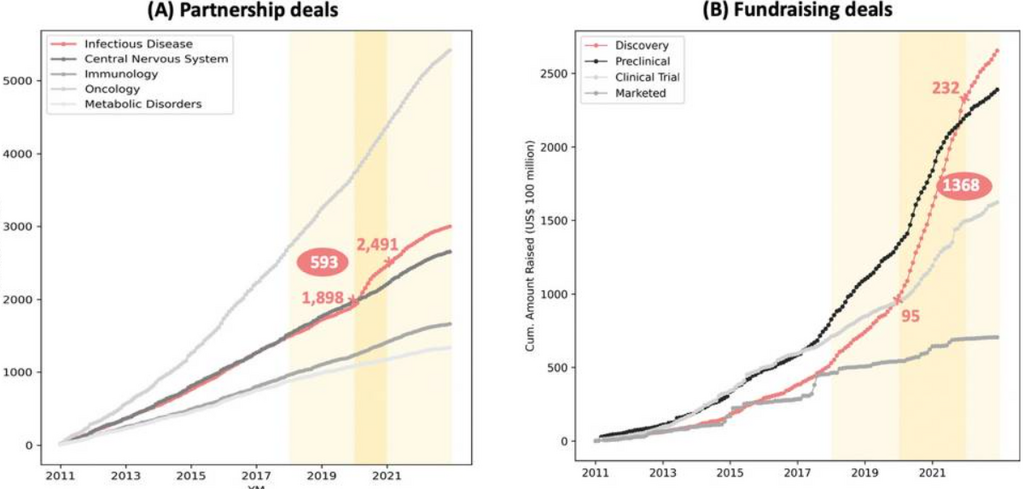
In 2020, substantial resources were directed toward projects related to SARS-CoV-2 with the aim of combating the virus. However, this trend had already commenced toward the close of 2019 when the initial waves of the virus started.
Our team of scientists have combined efforts to evaluate if inhalation based delivery of nebulized neutralizing antibodies could be the potential game changer in the medical field when it comes to reducing viral load in the respiratory system.
Using the anti-viral antibodies as a starting point, this study has given us a way to learn more about fighting other current and potentially emerging respiratory viruses too.
Monoclonal antibodies have proven themselves as good therapeutics in multiple applications as well as against the SARS-CoV-2 virus. But as new variants of the virus keep emerging and causing worry, most of the developed antibodies show significantly decreased or no virus-neutralization capacity.
Additionally, administering these antibodies through inhalation, provides a high concentration of antibodies in the respiratory tract, making it a good alternative for the current systemic administration.
Concentrations of systemically administered antibodies in the mucosal epithelium, a primary site of initial infection, are dependent on neonatal Fc receptor mediated transport and require high systemic drug concentrations.
Like all other biotech, pharma and academic labs, we initiated in 2020 an extensive program for developing SARS-CoV-2 neutralizing antibodies, but soon were humbled by nature as the mutational plasticity of the virus started to evolve as it was spreading throughout the populations.
Antibody obtained from the recovered patient’s serum proved to be efficient at first, but lost its neutralization properties after Beta variant emerged, rendering it useless. To adapt, we discovered antibodies from immunized rabbits and found a canditate with unique properties.
This antibody has the ability to bind to a region of the virus that has stayed the same since, so it can work against many variants of the virus.
To reduce the viral load more effectively in the lung, the team developed an inhalable formulation of the SARS-CoV-2 neutralizing antibody binding to a conserved epitope on the Spike protein, ensuring pan-neutralizing properties.
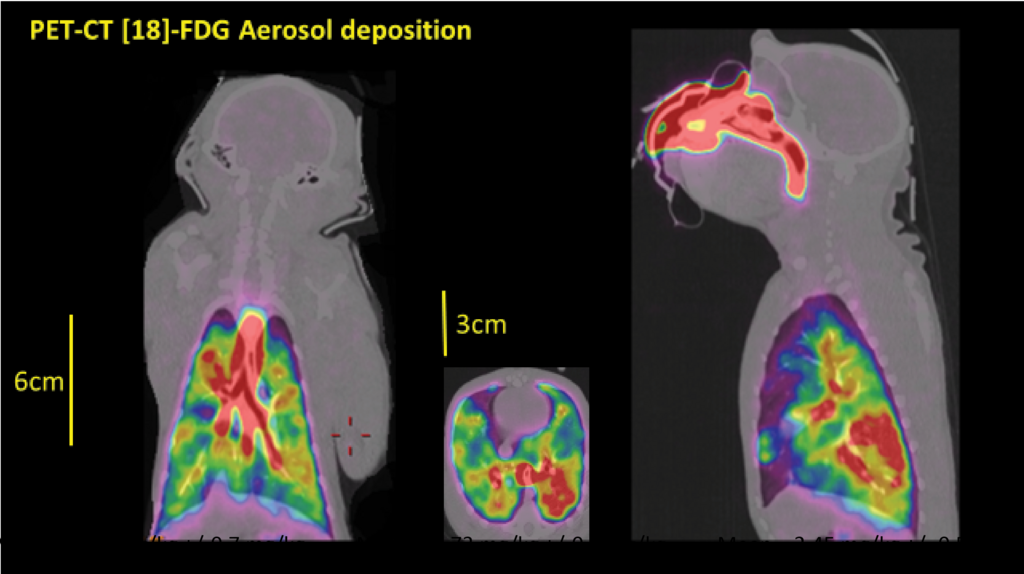
Nebulization is not an unambiguous process, meaning the outcome differs from molecule to molecule. Similar to the current use of intravenous vaccines, the results may vary depending on the amount of antibodies produced by the individual’s immune system.
However, administration of this antibody via a vibrating mesh nebulization device retained antibody integrity and resulted in effective distribution of the antibody in the upper and lower respiratory tract of non-human primates (NHP).
Meaning in comparison with intravenous administration, significantly higher antibody concentrations can be obtained in the lung, resulting in highly effective reduction in viral load post SARS-CoV-2 challenge.
In even simpler terms, this means that the antibodies spread more effectively through the upper and lower airways and compared to administering into the vein.
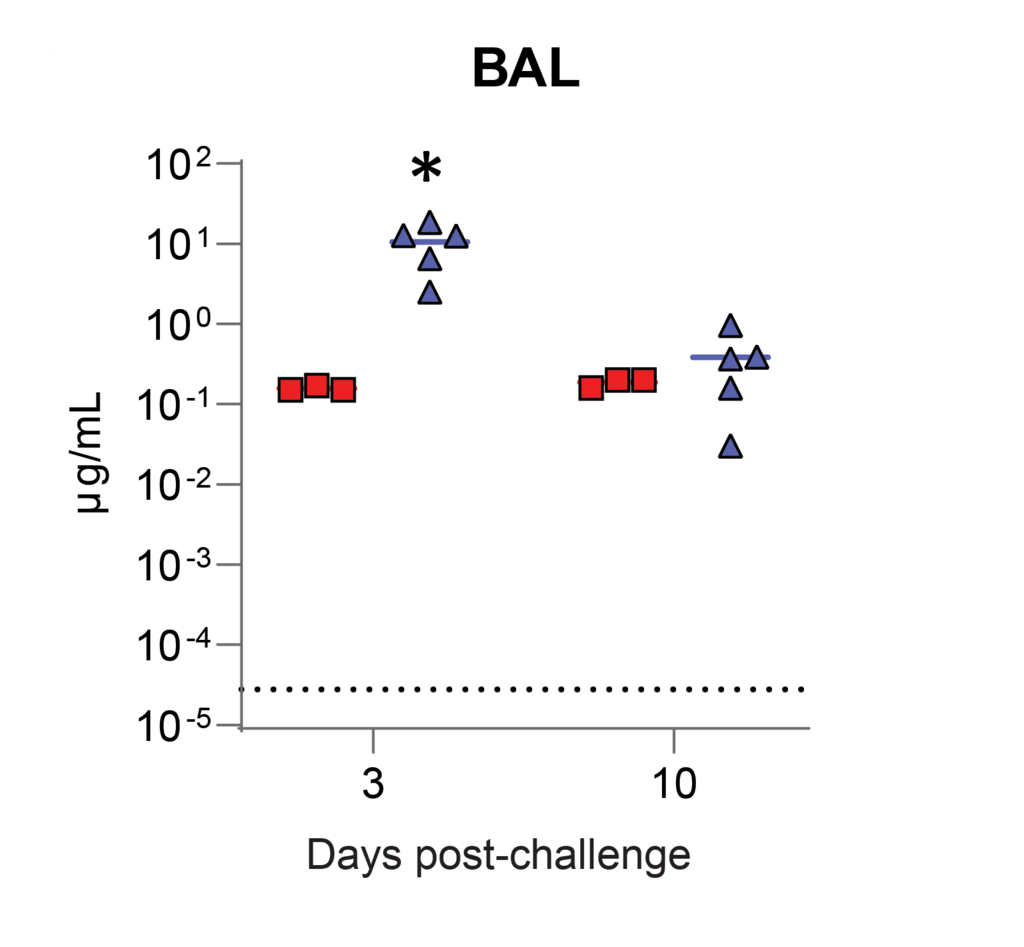
As shown on Figure 6, nebulization for antibody delivery, has a higher yield of antibodies in the lungs. Squares present antibodies found using the intravenous method, triangles using nebulization.
This approach may reduce the barriers of access and uptake of antibody therapeutics in real-world clinical settings. And more, provide a more effective blueprint for targeting existing and potentially emerging respiratory tract viruses.
Icosagen is focused on reaching scientific breakthroughs that could positively impact medical well-being and has worked on anti-Covid research since 2020.
Despite the attention to COVID-19 having subsided, the risk of the virus reemerging or mutating to other potentially lethal variants is enough to deem the research relevant even today.
“The emergency situation of the pandemic is over, but I received an unexpected reminder last week that the virus is still out there by getting hit by it a second time now.
While I was luckily non-functional for only a couple of days and recovered fast, there are populations for which the virus can cause significant morbidity and mortality,” commented our Chief Scientific Officer Mart Ustav Jr. on his LinkedIn post.
Scientific breakthroughs and keeping an innovative mindset is the key to advancing technologies. Whether to fight against COVID-19 or other future respiratory viral threats through pioneering research and collaborations with the global scientific community.
"While I was luckily non-functional for only a couple of days and recovered fast, there are populations for which the virus can cause significant morbidity and mortality"
However, it's important to know that this project has been a team effort.
“The scientific know-how our collaborators have is amazing. This study has been long in the works and the results show effort,” said our researcher and project leader Paule Hermet.
Thank you again, CEA, University of Toronto, University of Tartu, York University, Norwegian University of Science and Technology (NTNU), Derek Wilson, Roger Le Grand, Benoit Delache, Cécile Hérate, Eva Žusinaite, Denis Kainov, Andres Merits and Oliver Ernst.
And, of course, our Icosagen team - antibody discovery team led by Gaily Kivi and our researcher Paule Hermet.
To get a more detailed look at the publication at hand, click here.
Written by HERMET P., DELANCHE B., HERATE C., WOLF E., KIVI G., JURONEN E., MUMM K., ŽUSINAITE E., KAINOV D., SANKOVSKI E., VIRUMÄE K., PLANKEN A., MERITS A., BESAW J. E., YEE A. W., MORIZUMI T., KIM K., KUO A., BERRICHE A., DEREUDDRE-BOSQUET N., SCONOSCIUTI Q., NANINCK T., RELOUZAT F., CAVARELLI M., USTAV M., WILSON D., ERNST O. P., MÄNNIK A., LEGRAND R., USTAV JR M. | Edited by ICOSAGEN STAFF | Publication
[1] Yu Tzu-Hui, Mei, Yung-Yui., Tseng, Yufeng Jane. "Biopharma innovation trends during COVID-19 and beyond: an evidence from global partnerships and fundraising activities, 2011-2022". Globalization and Health, no. 19 (August 2023): 17.