Search from website
Search from website
Home CRDMO Services Quality Control
Icosagen offers a broad range of tailored services for the development and production of recombinant antibodies, including complex antibody formats.
Though the challenge is with the latter, as many hard-to-express antigens also require very customized approach to purification and storage buffer formulation. For each step Icosagen has highly qualified personnel focused on solving any challenges.

Recombinant antibody production services includes the possibility to change antibody subtype or host specificity, and to generate various types of antibodies, such as human IgG1, IgG2, IgG4, mouse IgG1, IgG2a, IgG2b, IgG3, rat IgG1, IgG2a, rabbit IgG, chicken IgY, chimeric antibodies, antibody fragments, single chain molecules, bispecific antibodies (bi- scFV-Fc, DVD-Ig, Crossmabs), etc.
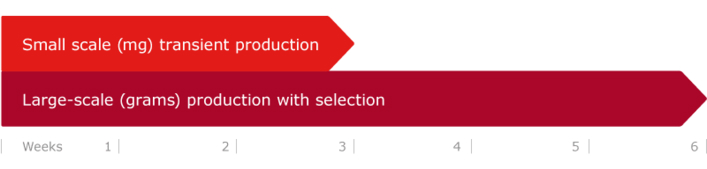
is used to deliver up to hundreds of milligrams of purified antibody within 3-4 weeks starting from vector cloning.
is used to scale up the production volumes up to 40 liters cost effectively, and to deliver multiple gram quantities of purified antibody. In this case, also episomal production cell bank can be generated, which allows to omit transfection completely when repetitive and constant supply of the same antibody is required with consistent reproducibility.
Synthesis of variable region DNA
Expression vector construction
Transfection and expression analysis
Timescale: 1 week if cDNA is available, 4 weeks if variable regions need to be synthesized
Deliverables: Expression/secretion data 48h transfection
Antibiotic selection. Production volume scale-up
Production cell bank generation
Protein production. Production analysis
Timescale: 3 weeks. In case of transient production (without selection and cell bank generation) — 1 week.
Deliverables: Antibody constaining supernatant, analysis data
Antibody purification
Antibody characterization
Timescale: 1 week
Deliverables: Purified antibody, QC data, characterisation data, detailed report



