Search from website
Search from website
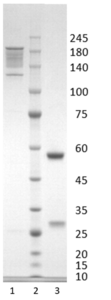
Figure 1. Simply Blue stained SDS-PAGE analysis of Monoclonal antibody to Influenza A, clone 8F8. 4-12% gradient gel is used for analysis. Lane 1. 0.8 µg Monoclonal antibody to Influenza A, clone 8F8 (-DTT) Lane 2. Size marker (Smobio) Lane 3. 0.8 µg Monoclonal antibody to Influenza A, clone 8F8 (+DTT).
Figure 2. HPLC analytical SEC for final product.
Figure 3. HPLC analytical SEC after 3 freeze-thaw cycles.
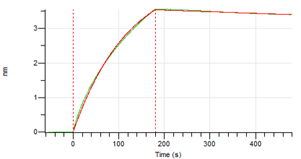
Figure 4.Octet RED96e analysis, antibody was loaded on sensor for capture of H3N2 nucleocapsid protein.
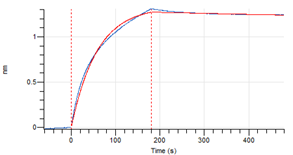
Figure 5. Octet RED96e analysis, antibody was loaded on sensor for capture of H1N1 nucleocapsid protein.
Catalogue #
R1-190-100
Name
mAb to Influenza A, clone 8F8
Target
Recombinant Influenza A Nucleocapsid protein
Target Description
Produced by CHO-based Icosagen Cell Factory Ltd. proprietary suspension cell line
Uniprot ID
P03466
Source
Human
Clonality
Human monoclonal
Clone
8F8
Class
hIgG1
Dissociation constant (KD)
7.50 x 10-09 M (measured against Influenza A H1N1 nucleocapsid protein)
4.05 x 10-09 M (measured against Influenza A H3N2 nucleocapsid protein)
Application
ELISA
Purification
Protein A affinity chromatography following gel filtration
Buffer
PBS pH 7.4
QC
Simply Safe Stained SDS-PAGE, analytical SEC, Octet binding
Shipping
Shipped with blue ice.
Storage
Store at +4 °C. Divide antibody into aliquots prior usage.
This product is for research use only
TECHNICAL ASSISTANCE
Please refer any technical questions to
technical.support@icosagen.com